

Securing a college education can be financially challenging, but scholarships offer a beacon of hope for many students. High school sophomores may feel it’s too early...


Alzheimer’s disease presents a myriad of challenges for patients and their loved ones, navigating the complex terrain of long-term care, emotional support, and financial management. It...


Modifying a parenting plan in Massachusetts can feel overwhelming. Life changes quickly. You deserve a plan that reflects your evolving needs. Boston family law attorneys can...


Navigating legal documents can feel overwhelming. You often face pages filled with terms that seem foreign. Yet, understanding these documents is crucial. Whether signing a lease,...


GTA 5 Online is one of the most dynamic and engaging open-world multiplayer games ever created. But anyone who’s spent a few hours in Los Santos...


Bible verse bracelets have become increasingly popular in recent years as people seek ways to carry their faith with them throughout the day. According to surveys...


In the toughest work environments—construction sites, workshops, farms, and fields—you need gear that doesn’t just survive the grind, but thrives in it. That’s where the work...


Facing an assault accusation in Dallas is daunting. You might feel stressed and uncertain, but you aren’t alone. Understanding the steps to take can help you...
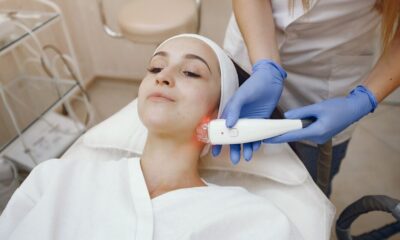

Over the past two decades, skin laser treatment has emerged as a revolutionary tool in the world of dermatology and aesthetic medicine. As people seek more...


Switching to clean energy is no longer just an option; it’s quickly becoming the standard for a sustainable future. Whether you’re a homeowner looking to reduce...