Advanced Immunomodulating Multi-receptor System
CYTO-200 AIMS™ Program
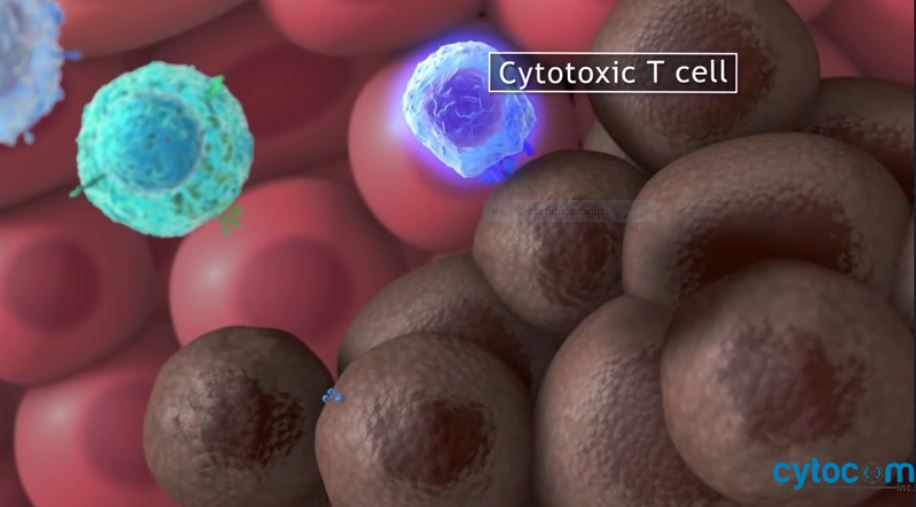
CYTO-200 program therapies, are noroxymorphone analogs developed by discovering the anti-angiogenic and anti-inflammatory properties of naltrexone, yielding a new way of fighting cancer, inflammatory, autoimmune and infectious diseases.
| Discovery | Lead Optimization | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | NDA |
Current CYTO-200 AIMS™ analogs are undergoing or have performed in numerous phased trials, including: Crohn’s Disease and COVID-19
Through the AIMS™ platform, we are expanding our understanding of the relationship between noroxymorphone analogs to determine how multiple factors impact pharmacokinetic-pharmacodynamic relationships, potency, and selectivity. Through methodical property analysis we have discovered, with more accuracy, targets with the improved properties to address specific patient needs eliciting immune response levels not achieved by other published immunotherapy
CYTO-400 AIMS™ Program
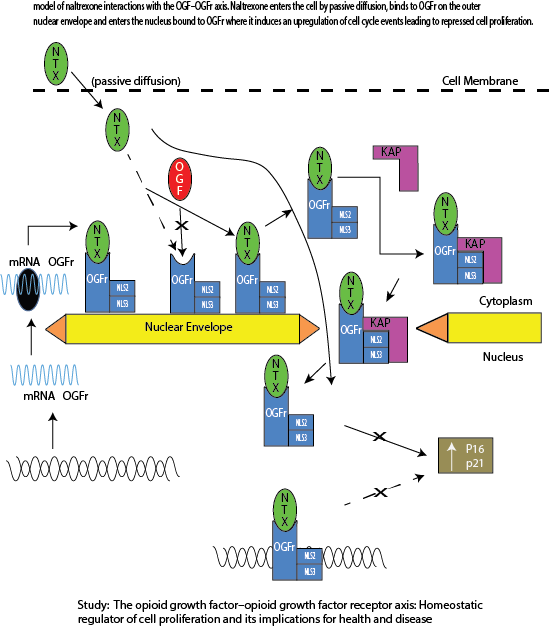
CYTO-400 program therapies are proenkephalin analogs developed to target multiple receptors associated with cancer, inflammatory, autoimmune, and infectious diseases.
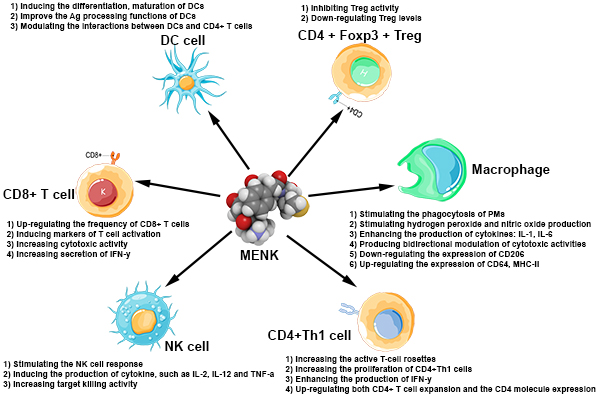
After years of work metenkepahlin, an endogenous neuropeptide which appears to have a crucial role in both neuroendocrine and immune systems, clinical studies in cancer patients have shown that metenkephalin activates immune cells directly and, by inhibiting regulatory T-cells (Tregs), may also change the tumor microenvironment by binding to opioid receptor on/or in cancer cells (Shan: Methionine enkephalin, its role in immunoregulation and cancer therapy). Through the AIMS™ platform, we are hopeful that proenkephalin analogs may exhibit many advantages including safety and a broad-spectrum of immunoregulatory and anticancer activities.
| Discovery | Lead Optimization | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | NDA |
Current CYTO-400 AIMS™ analogs are undergoing or have performed in numerous phased trials.
CYTO-600 TLR5 Program
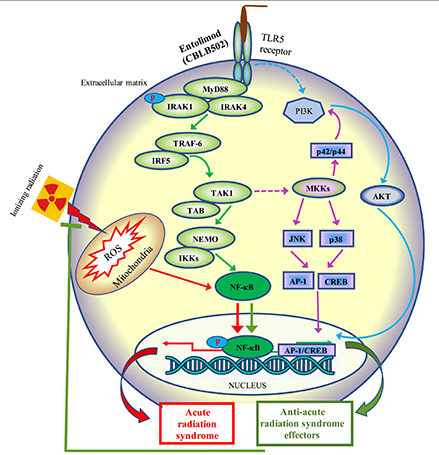
CYTO-600 TLR5 program therapies are analogs developed to target multiple indications such as: hematology, acute radiation syndrome, cancer, inflammatory, autoimmune, and infectious diseases with a focus on TLR5. CYTO-600 TLR5 is a recombinant protein that acts as an agonist of toll-like receptor 5 (TLR5), an innate immunity receptor. CYTO-600 activation of TLR5 triggers NF-kB signaling, mobilizing an innate immune response that drives expression of numerous genes, including inhibitors of apoptosis, scavengers of reactive oxygen species, and a spectrum of protective or regenerative cytokines.
| Discovery | Lead Optimization | Pre-Clinical | Pivotal Animal Studies | Human Saefty / Dose Conversion |
| Discovery | Lead Optimization | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | NDA |
CYTO-601 ARS: Cytocom is hopeful that the CYTO-600 efficacy, safety and animal-to-human dose conversion data should provide for accelerated and favorable communication with FDA.
CYTO-602 Oncology: The CYTO602 program looks to leverage the data obtained from numerous trials and studies conducted with TLR5 variants over the years, including advanced solid tumors, lung, colorectal, lymphoma, breast, uveal melanoma and bladder. Studies have shown the potential to modify the microenvironment in liver, lungs, and bladder to suppress TLR5+ and TLR5- metastases, generate an adaptive immune response for prolonged antitumor effect, and protect from radio and chemotherapy side effects.
Additionally, there is a strong case for CYTO-600 use in improving palliative radiation therapy by improving efficacy and strengthening systemic immunotherapeutic effects.
We will look to initiate further TLR5 variant studies both as monotherapy and adjuvant.
CYTO-604 Vaccine Adjuvants (formerly SA-702):is a new therapeutic approach with entolimod that employs the immunopotentiating properties of the drug together with alum (aluminum salts) as a vaccine adjuvant. In this context, CYTO-600’s immune activity would be harnessed to enhance the efficacy of vaccines by eliciting a stronger immune response to the vaccine’s particular antigen.
Many vaccines require an adjuvant to induce sufficient immune response. It is estimated that about one half of 30 of the most common vaccines approved by FDA contain alum as an adjuvant. Until recently, alum was the only adjuvant approved by FDA, but often alum alone does not allow new vaccines to reach sufficient clinical potency.
A shortage of effective and safe adjuvants is a major bottleneck in vaccine development. A newer generation of vaccine boosters combine classic adjuvants mixed with immunomodulators (like entolimod). We have collaborated with academic investigators who have performed preclinical studies that support the adjuvant potential of CYTO-604s in enhancing vaccine immune and wish to translate these data to clinical studies to document the immunopotentiating effect of the drug.